
Structure and mechanics of Cytoskeletal filaments
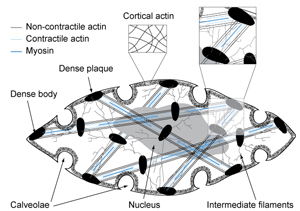 Cells must be able to bear, transmit, sense, and respond to forces. Failure to either properly react to external stresses is known to lead to several pathological cardiovascular conditions, including hypertension and smooth muscle hypertrophy. Here our overall goal is to understand the nature and regulation of cytoskeletal dynamics and mechanics. Actin and its associated actin-binding proteins (ABPs) are key mechanical components of the cytoskeleton and are able to dynamically modulate cell mechanics through remodeling. We study the effects of ABPs on actin structure and dynamics using a custom-built total internal reflectance fluorescence (TIR) microscope and confocal microscopy (Collins et al., 2011; Jensen et al., 2012).We also determine the impact of vascular smooth muscle cytoskeletal actin-binding proteins (ABPs) on cytoskeletal filament structure and mechanics. We focus on characterizing actin filament mechanics and dynamics as well as cell mechanics. To achieve this goal we collaborate with Dr. Lehman at Boston University to carry out high-resolution electron microscopy, helical reconstruction, and single particle analysis of reconstituted thin filaments to define the molecular interactions of ABPs on actin. Corresponding strategies in our lab use laser-trapping and single molecule fluorescence methods to determine the effects of force on ABP-binding to F-actin and the effect of ABPs on the mechanical performance of thin filaments (Schmidt et al., 2015; Jensen et al., 2014; Greenberg et al., 2008). Collaborative studies with the Dr. Weitz at Harvard University on crosslinked actin networks using bulk rheology are being used to characterize the effects of ABPs in a system that more closely mimics a cytoskeletal actin network (Guo et al., 2013; Jensen et al., 2014).
Cells must be able to bear, transmit, sense, and respond to forces. Failure to either properly react to external stresses is known to lead to several pathological cardiovascular conditions, including hypertension and smooth muscle hypertrophy. Here our overall goal is to understand the nature and regulation of cytoskeletal dynamics and mechanics. Actin and its associated actin-binding proteins (ABPs) are key mechanical components of the cytoskeleton and are able to dynamically modulate cell mechanics through remodeling. We study the effects of ABPs on actin structure and dynamics using a custom-built total internal reflectance fluorescence (TIR) microscope and confocal microscopy (Collins et al., 2011; Jensen et al., 2012).We also determine the impact of vascular smooth muscle cytoskeletal actin-binding proteins (ABPs) on cytoskeletal filament structure and mechanics. We focus on characterizing actin filament mechanics and dynamics as well as cell mechanics. To achieve this goal we collaborate with Dr. Lehman at Boston University to carry out high-resolution electron microscopy, helical reconstruction, and single particle analysis of reconstituted thin filaments to define the molecular interactions of ABPs on actin. Corresponding strategies in our lab use laser-trapping and single molecule fluorescence methods to determine the effects of force on ABP-binding to F-actin and the effect of ABPs on the mechanical performance of thin filaments (Schmidt et al., 2015; Jensen et al., 2014; Greenberg et al., 2008). Collaborative studies with the Dr. Weitz at Harvard University on crosslinked actin networks using bulk rheology are being used to characterize the effects of ABPs in a system that more closely mimics a cytoskeletal actin network (Guo et al., 2013; Jensen et al., 2014).
We are now extending this work to intact cells. In collaboration with the Morgan lab we use custom-built magnetic tweezers to measure the mechanics and dynamic responses of the cytoskeleton to externally applied forces (Saphirstein et al., 2013). We are also using optical tweezers designed and constructed in the lab to manipulate injected beads in the cell interior to quantify the local mechanical environment in terms of the storage and loss moduli. Recent collaborative work with the Weitz lab provides critical information for how the cell, despite being a largely elastic material, can allow passive transport of objects much larger than the network mesh size (Guo et al., 2013; Guo et al., 2014).
Associated Publications
Schmidt WM, Lehman W, Moore JR. (2015) Direct observation of tropomyosin binding to actin filaments. Cytoskeleton (Hoboken). May 29. doi: 10.1002/cm.21225. [Epub ahead of print] PMID: 26033920
Guo M., Ehrlicher A.J., Jensen M.H., Renz M., Moore, J.R., Goldman R.D., Lippincott-Schwartz J., Mackintosh F.C., Weitz D.A. (2014) Probing the stochastic motor-driven properties of the cytoplasm using force spectrum microscopy. Cell. 158(4):822-832
Jensen M.H., Morris E.J., Gallant C. M., Morgan K.G., Weitz D.A., Moore, J.R. (2014) Mechanism of calponin stabilization of crosslinked actin networks Biophys. J. 106:793-800 PMCID: PMC3944828
Saphirstein R. J., Gao Y.Z., Jensen M.H., Gallant C.M., Vetterkind S., Moore, J.R., Morgan K.G.(2013) The focal adhesion: a regulated component of aortic stiffness. PLoS One. 23;8(4):e62461. doi: 10.1371/journal.pone.0062461. PMCID: PMC3633884.
Guo M., Ehrlicher A.J., Mahammad S., Fabich H., Jensen M.H., Moore, J.R., Fredberg J.J., GoldmanR.D., Weitz D.A. (2013) The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys J. 2013 105(7):1562-8. PMCID: PMC3791300.
Jensen M.H., Morris E.J., Huang R., Rebowski G., Dominguez R., Weitz D.A., Moore, J.R., Wang C-L. (2012) The conformational state of actin filaments regulates branching by actin-related protein 2/3 (Arp2/3) complex. J Biol Chem. 287(37):31447-53 PMCID: PMC3438974
Jensen M.H., Watt J., Hodgkinson J., Gallant C., Appel S., El-Mezgueldi M., Angelini T., Morgan K.G., Lehman W., and Moore, J.R. (2011) Effects of basic calponin on the flexural mechanics and stability of F-actin CytoskeletonCytoskeleton 69(1):49-58.PMCID: PMC3355516.
Collins A., Huang R., Jensen M. H., Moore, J.R., Lehman W., Wang Chih-Lueh Albert (2011) Structural studies on maturing actin filaments. BioArchitechture. BioArchitechture. 1:127-33.PMCID: PMC3173961.
Greenberg, M., Wang, C-L., Lehman, W., Moore, J., (2008) Modulation of actin mechanics by caldesmon and tropomyosin. Cell Motility and Cytoskeleton. Feb;65(2):156-64. PMCID: PMC2975105.