|
-
Experiment
14
-
Double
Displacement Reactions
Overview
In this experiment you ultimately want
to try to identify two "unknown" solutions of ionic substances, by
comparing the reactions of the "unknown" substances with the reactions
of a set of "known" substances.
When a chemical reaction
takes place, there is usually some sort of observable evidence
for the reaction: a gas may be produced (causing bubbling); the
color of the sample may change; an odor may be noticed; heat may be
evolved. Perhaps the most common bit of evidence that a reaction has
taken place, however, is the formation of a solid precipitate
when the reactants are mixed. For example, if clear, colorless
solutions of barium nitrate [Ba(NO3)2] and
sodium sulfate (Na2SO4) are mixed, a
precipitate of barium sulfate, BaSO4 immediately forms:
| |
|
Ba(NO3)2(aq)
+ Na2SO4(aq) 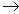 2NaNO3(aq)
+ BaSO4(s) 2NaNO3(aq)
+ BaSO4(s) |
| |
This reaction is an
example of a general class of reactions called double
displacement (or metathesis) reactions. Such reactions
have the general format
| |
|
A+B-(aq)
+ X+Y-(aq) 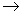 A+Y-(aq) + X+B-(s)
A+Y-(aq) + X+B-(s)
|
| |
In a sense, each positive ion in the
reactants has "displaced" the other positive ion. One of the new
combinations of ions is insoluble in water and forms a
precipitate. The precipitate could be filtered, and the water
could be evaporated from the ions remaining in solution, to give two
new substances. In this experiment you will use the appearance (or
nonappearance) of a precipitate when two reagents are mixed
as a means of identifying your two unknown solutions.
The most important part of
this experiment is learning to relate the Table of Solubilities on
Page 106 with your experimental observations. For example, suppose
you mixed together CaCl2 solution with AgNO3
solution: a precipitate forms. But what is the identity of
the precipitate?? The two possible products of the reaction are AgCl
and Ca(NO3)2. Find the intersection of Ca2+
and NO3- in the Table of Solubilities: there
is an "S" there, which means that Ca(NO3)2
is soluble and must not be the substance that precipitated.
However, if you look at the intersection of Ag+ and Cl-,
there is an "I" there, which means that AgCl is insoluble and
therefore must be the precipitate that formed.
Data
Your data in Parts I and
II on Page 109 should reflect your observations of the solutions you
have available for the experiment and their mutual reactions when
systematically combined. In particular, make certain that you note
the color of the individual solutions (e.g., NiSO4 is
bright green, whereas CuSO4 is bright blue). Be sure you
understand the difference between saying a solution is "clear"
(meaning there are no cloudy solids present) and "colorless"
(which means that the solution shows no apparent inherent color). In
Part II, record carefully your observations of what happens when you
mix two solutions together. If nothing happens, write "no reaction".
If a precipitate or cloudiness forms, describe the color and texture
of the precipitate. For example, when NiSO4 (itself a
green solution) reacts to form a precipitate, is the resulting
precipitate green or is the supernatant liquid above the precipitate
green? Since the green color is due to the presence of the Ni2+
ion, where the green color ends up can help you in identifying
things.
Chemical Equations
Page 110, Part III
In this part of the
experiment, you have to write the balanced overall and net
ionic equations for the reaction that resulted in precipitate
formation. To do this, you need to apply the Table of Solubilities
on Page 106 as described in the Overview above. When you look
at the Table of Solubilities to determine the formula of the
precipitate, realize that this table essentially gives you
the net ionic equation for the reaction. For example, for the
reaction given earlier
| |
|
Ba(NO3)2(aq)
+ Na2SO4(aq) 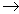 2NaNO3(aq) + BaSO4(s)
2NaNO3(aq) + BaSO4(s)
|
| |
I knew that BaSO4
was the precipitate by looking up the intersection of Ba2+
and SO42- in the Table of Solubilities. The
net ionic reaction for this overall reaction is just what I do in my
head when looking in the Table of Solubilities: "barium ion plus
sulfate ion gives an insoluble precipitate of barium sulfate", which
gives the net ionic equation
| |
|
Ba2+(aq)
+ SO42-(aq) 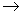 BaSO4(s)
BaSO4(s) |
| |
Page 111, Part IV
Identification of your two unknown
solutions requires your looking back at your data for the known and
"extra" solutions, and also checking frequently the Table of
Solubilities. You are told on Page 107 the possible positive and
negative ions in the unknowns-only these ions have been used. You
can determine the identity of your unknowns by a "process of
elimination". First of all, there are many substances which can be
excluded as possibilities for what the unknowns are. Looking
at the Table of Solubilities, you will see that there are many
combinations which are insoluble in water. If a substance is
insoluble in water, then we couldn't have given you a
solution of the substance as an unknown!! This may seem obvious,
but you'd be surprised how many students report insoluble substances
as their unknown's identity.
The positive ions listed on Page 107 as
being candidates for the unknowns are Ba2+, Pb2+,
Ag+, and Ni2+. As mentioned earlier, Ni2+
solutions are bright green, so if your unknown is not green, it
cannot contain Ni2+. So let's say we have a colorless
unknown, and perform reactions of the unknown with each of the known
and "extra" solutions. Since there are only four possible positive
ions, just go through the Table of Solubilities and your reaction
observations trying to make a match.
Suppose you thought the positive ion in
your unknown might be either Ba2+ or Pb2+. For
example, Ba2+ and Pb2+ both form precipitates
with CrO4-. However, Pb2+ forms a
precipitate with Cl-, whereas Ba2+ does not.
If your unknown formed a precipitate with Cl-, then, of
the two positive ions considered, it probably is Pb2+.
Take notes as you go
through the data, and treat it like a puzzle. It may not be even
possible to completely identify both ions in the unknowns, but as
long as your observations, reasoning, and conclusions are correct,
you should be able to narrow things down to a few possibilities at
the least. You will receive credit not for just the right answer,
but for how you arrived at the answer. Guessing does not help!
Reasoning things out is the way to go.
Questions
After identifying your
unknowns, writing a few more equations should be a piece of cake.
Just use the Table of Solubilities to identify the precipitate in
each case.
General Solubility Rules
In addition to the Table
of Solubilities in your lab manual, there are some "general
solubility rules" that you might find helpful in identifying your
unknown. There is a list of these in your textbook, or you can refer to the list below
taken from
http://www.csudh.edu/oliver/chemdata/solrules.htm
-
Salts
containing Group I elements are soluble (Li+,
Na+, K+, Cs+, Rb+).
Exceptions to this rule are rare. Salts containing
the ammonium ion (NH4+) are
also soluble.
-
Salts
containing nitrate ion (NO3-)
are generally soluble.
-
Salts
containing Cl -, Br -, I
- are generally soluble. Important exceptions
to this rule are halide salts of Ag+, Pb2+,
and (Hg2)2+. Thus, AgCl, PbBr2,
and Hg2Cl2 are all insoluble.
-
Most
silver salts are insoluble. AgNO3 and
Ag(C2H3O2) are
common soluble salts of silver; virtually anything
else is insoluble.
-
Most
sulfate salts are soluble. Important exceptions to
this rule include BaSO4, PbSO4,
Ag2SO4, and CaSO4.
-
Most
hydroxide salts are only slightly soluble. Hydroxide
salts of Group I elements are soluble. Hydroxide
salts of Group II elements (Ca, Sr, and Ba) are
slightly soluble. Hydroxide salts of transition
metals and Al3+ are insoluble. Thus,
Fe(OH)3, Al(OH)3, Co(OH)2
are not soluble.
-
Most
sulfides of transition metals are highly insoluble.
Thus, CdS, FeS, ZnS, Ag2S are all
insoluble. Arsenic, antimony, bismuth, and lead
sulfides are also insoluble.
-
Carbonates
are frequently insoluble. Group II carbonates (Ca,
Sr, and Ba) are insoluble. Some other insoluble
carbonates include FeCO3, PbCO3.
Carbonates become soluble in acid solution.
-
Chromates
are frequently insoluble. Examples: PbCrO4,
BaCrO4
-
Phosphates
are frequently insoluble. Examples: Ca3(PO4)2,
Ag3PO4
-
Fluorides
are frequently insoluble. Examples: BaF2,
MgF2 PbF2.

|