|
|
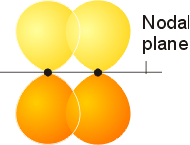
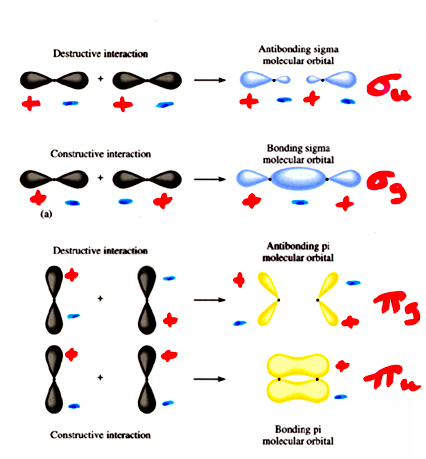
Adjacent atomic orbitals can also overlap side-by-side. This type of constructive overlap results in electron density above and below the internuclear axis, and is called a p bond. Neighboring px or neighboring py orbitals can overlap in this way. A nodal plane which passes through the internuclear axis results.
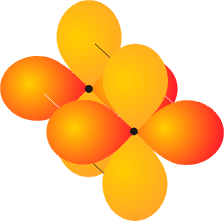
Atomic orbitals on neighboring atoms can also overlap face-to-face. This occurs between d orbitals, and is seen in transition metal compounds. The bond, which is called a d bond, results in two nodal planes which intersect along the internuclear axis.
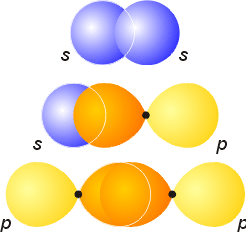
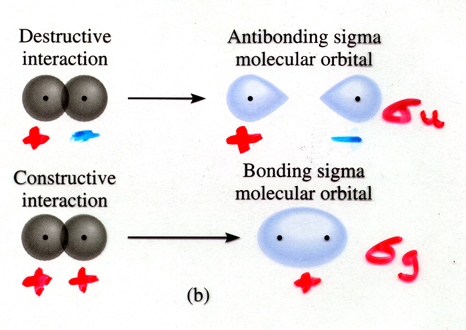
Energy level diagrams can be created for molecular orbitals, and they are similar to atomic energy level diagrams. Bonding orbitals (constructive overlap) are always lower in energy than their antibonding (destructive overlap) counterparts. Each level is designated by its number, type (s,p,d), and its symmetry with relation to inversion through the center of the molecule. If a molecular orbital is symmetrical with respect to inversion, it has a subscript g (gerade, for even). If it is asymmetrical with respect to inversion, it is given a subscript u (ungerade, for uneven). The molecular orbitals for the combination of s orbitals on neighboring atoms are drawn below. The bonding orbital is given the designation sg, and will be lower in energy. The antibonding orbital, which will be higher in energy, is given the designation su, because the sign of the orbital changes upon inversion through the center.
The possible combinations between neighboring p orbitals are shown below. The orbitals can combine end-to-end to form bonding and antibonding s orbitals, or side-by-side to produce bonding and antibonding p orbitals. It is important to note that the notations of gerade and ungerade are unrelated to the bonding or antibonding nature of the resulting molecular orbital. Each type of orbital and its symmetry must be considered separately.
The above molecular orbitals differ in energy, and an energy level diagrams can be constructed for simple diatomic molecules. The simplest diagram, which assumes no interaction between s orbitals on one atom and p orbitals on the other has the following form: (Please note that the numerical prefixes for the orbitals are not correct. The lowest orbital should be 1sg, and the p orbitals in the upper section should both have the prefix 1p - see figure 3.14 of the text.)
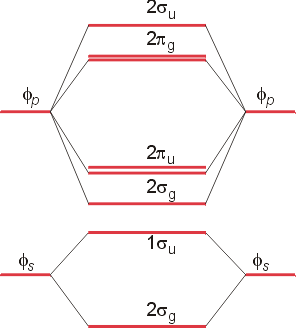
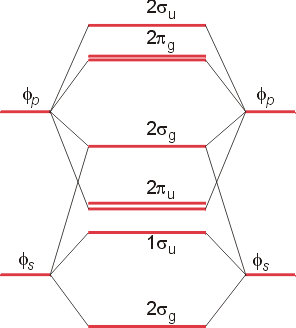
The lower orbitals represent the sigma bonding and antibonding orbitals. The upper section shows the sigma (end-to-end) bonding orbital between two pz orbitals (lowest in energy), then the two degenerate p bonding orbitals. Next in energy are the two degenerate antibonding p orbitals, and highest in energy, the s antibonding orbital. This type of diagram, which assumes that the 2p orbitals are high enough in energy so that they do not interact with the 2s orbitals, is appropriate for the diatomic molecules O2 and F2. For the diatomic molecules Li2 through N2, there is evidence for significant interaction between the 2p and the 2s orbitals. The energy diagram will be altered as follows: (Please note that the numbering is again incorrect. Your text contains the correct numbering for the orbitals - see figure 3.18)
Molecular orbital diagrams can also be developed for heteronuclear diatomic molecules. For these molecules, the overlap is often less, since the energy levels of the orbitals on each atom usually differ. The simplest case is for a molecule such as HF, where only the 1s orbital of hydrogen need be considered. It can overlap with both the 2s and 2pz orbitals on fluorine to produce the following MO diagram.
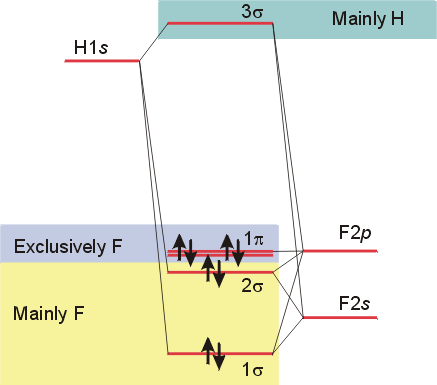
Note that the notation
gerade
and
ungerade are no longer used, as the molecule no longer has a center
of inversion. Since the orbitals on fluorine are lower in energy
than those of hydrogen, the bonding orbitals reside mostly on fluorine.
This is consistent with the high electronegativity of fluorine. The
p
molecular orbitals are non-bonding, and represent the lone pairs of electrons
in the px and py orbitals of fluorine. The
molecule can be viewed as having bonds resulting from the overlap of the
hydrogen 1s orbital with both the 2s and 2p orbitals of fluorine, or with
sp hybrid orbitals on fluorine. The 3s
molecular orbital, which is antibonding, resides predominantly on hydrogen,
because it is closer in energy to the orbitals on the hydrogen atom.
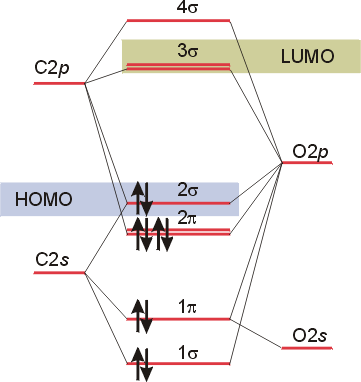
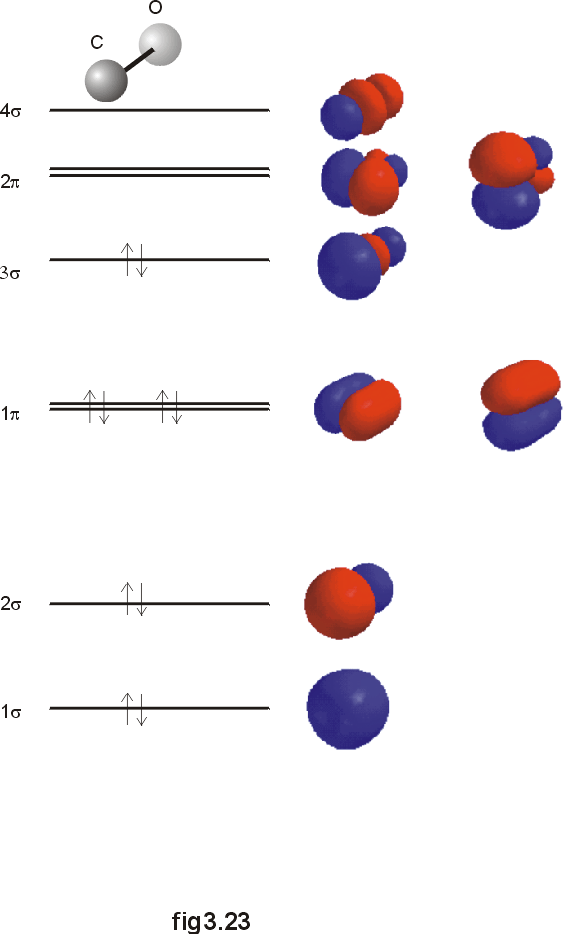
A similar treatment
for the bonding in carbon monoxide is significant, as CO serves a ligand
in many transition metal complexes. Here, the energies of the orbitals
in carbon and oxygen are a bit closer, so more interaction is possible.
Copyright ©1998 Beverly J. Volicer and Steven F. Tello, UMass Lowell. You may freely edit these pages for use in a non-profit, educational setting. Please include this copyright notice on all pages.