Transition from “catch” bonds to “slip” bonds in cell-adhesion systems¶
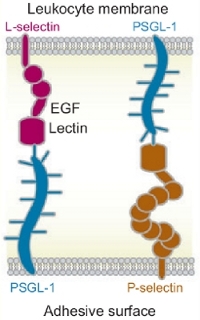
Figure: Structures of selectins, PSGL-1 and the receptors to which they bind on other cells.
Biological adhesion is at the intersection of material science, biology, and nanotechnology. It turns out that the bond lifetimes of some of the most biologically important adhesive bonds, called “catch” bonds, are enhanced mechanically, and only when shear stress exceeds some critical value (i.e. shear threshold) these bonds become weeker and break (“slip” bonds). Blood proteins called selectins, which mediate shear-enhanced adhesion of leukocytes rolling on the endothelial surface along the direction of blood flow, are capable of forming both “catch” bonds and “slip” bonds (see Figure). Consequently, in a blood stream leukocytes roll along the endothelial surface as receptor-ligand bonds form and break quickly when the shear rate (shear stress) exceeds the threshold, but they detach from the surface and move freely below a shear threshold. To this date, at least two types of adhesive proteins have been shown to form catch bonds—blood proteins called selectins and a bacterial protein called FimH. Although the phenomenological two-state kinetic model has been proposed by many authors to explain this counter-intuitive biphasic behavior, i.e. existence of the catch-regime for unbinding at a lower level of mechanical stress and the slip-regime at higher mechanical perturbation, there is no understanding of the microscopic molecular origin of the catch-slip duality. It is not known what triggers the dual response of cell-adhesion complexes to the external mechanical factors. We believe that the ability to control biological adhesion at a cellular level will also lead to interesting new developments in material science and biotechnology.
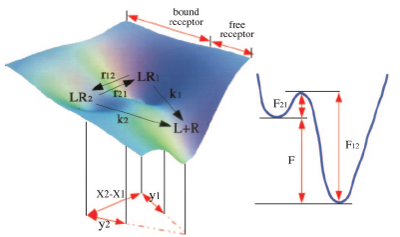
Figure: The energy landscape for the P-selectin-PSGL-1 complex (left panel). The 2D-profile shows the free energy projection along the conformational transitions (right panel).
It has become clear that there should be (at least) two bound states in the P-selectin-PSGL-1 complex, which differ in their mechanical stability agains the external factors. Indeed, we might visualize that an applied mechanical force tilts the free energy landscape, which governs the ligand-receptor (P-selectin-PSGL-1) interactions. This might result in a shift of the force-free equilibrium between the populations of the two bound forms of the complex, \(LR_1\) and \(LR_2\). Consequently, an increase in applied force might lead to a substantial re-distribution of the population from the high-affinity bound state at a low force, \(LR_1\), to the low-affinity bound state (at a low force), \(LR_2\), (see Figure). Hence, the force-induced alteration in the free-energy landscape is dynamically coupled to forced unbinding. We employ the GPU-based computing and numerical algorithms for MD simulations in implicit water, developed in our group, to perform the computational and theoretical studies of the force-induced dissociation of the “catch” bonds and “slip” bonds. We use the P-selectin-PSGL-1 cell-adhesion complex as a suitable model system (the X-ray structure for the monomeric P-selectin-PSGL-1 complex is the only resolved structure). We attempt to resolve the structural basis and to unmask the molecular mechanism which triggers a dual (either catch or slip) response of the P-selectin-PSGL-1 complex to the mechanical perturbation.