|
|
The elements have been classified an organized on the periodic table. The electron configuration for most elements can be easily obtained from a standard periodic table if the table is visualized as being divided into s, p, d, and f regions as illustrated below.
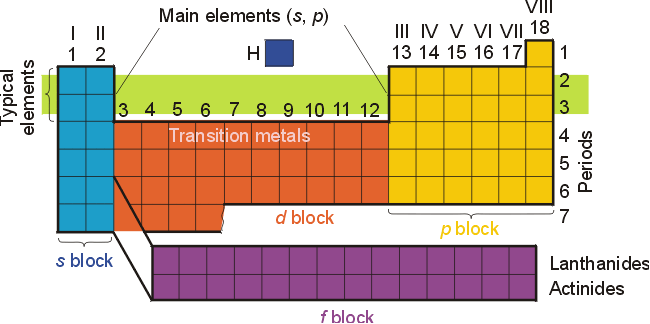
The relative positions
of the elements, and a thorough understanding of atomic orbitals and electron
configuration can lead to many generalizations concerning a given element's
properties and chemical behavior. For example, metals usually react
with non-metals to form hard crystalline (ionic) solids. When metals
react with other metals, the resulting alloy will have properties similar
to the two metals involved. Non-metals react with non-metals to form
volatile compounds.
A qualitative understanding
of wave functions for the atomic orbitals and their properties is essential
for explaining a variety of atomic properties such as size, ionization
energy, electron affinity, electronegativity and polarizability.
The nature of each type of atomic orbital and it's proximity and penetrating
ability towards the nucleus are key to understanding the energies of atomic
orbitals and the resulting electron configurations.
Properties of Atomic Orbitals
There are a few key
features of atomic orbitals which will help you understand the properties
of atoms. Of particular interest are the amplitude of the radial
wave functions near the nucleus, the number of radial nodes (locations
where the radial wave functions pass through zero), and the radial distribution
functions of the orbitals. [The radial distribution function
illustrates the probability of finding an electron a given distance from
the nucleus.] Although atoms contain many protons and electrons,
the basis of all wave functions comes from hydrogenic orbitals,
or orbitals for atoms with only one electron. It is important to
note that only the value of n, the principal quantum number, dictates the
energy of orbitals in hydrogenic atoms (or ions). Thus, the 2s and
2p orbitals are degenerate, having identical energies. Likewise,
the 3s, 3p and 3d orbitals will be degenerate. We know that in multi-electron
atoms, these orbitals have different energies. We must consider the
wave functions of the orbitals, and their proximity to the nucleus.
Orbitals which can penetrate closer to the nucleus will have lower energies
than those which are at greater distances from the nucleus.
Certain characteristics
of s orbitals are unique. All s orbitals have a finite (non zero)
amplitude at the nucleus, as seen in the radial wave functions. Other
orbitals have a zero amplitude at the nucleus. This results in significant
electron density close to the nucleus in all s orbitals.
A second feature
is that of radial nodes. All orbitals decay exponentially at sufficiently
great distances from the nucleus, but some oscillate through zero.
These oscillations are called radial nodes. The number of
nodes = n-l-1
(where n=principal quantum number, l
=
orbital angular momentum quantum number). For a 1s orbital, n=1,
l=0, and there are no radial nodes. A 2s orbital had 2-0-1 or 1 radial
node. For a 2p orbital, there are 2-1-1 or no radial nodes, whereas
a 3p orbital has 1 radial node. These features are illustrated below.
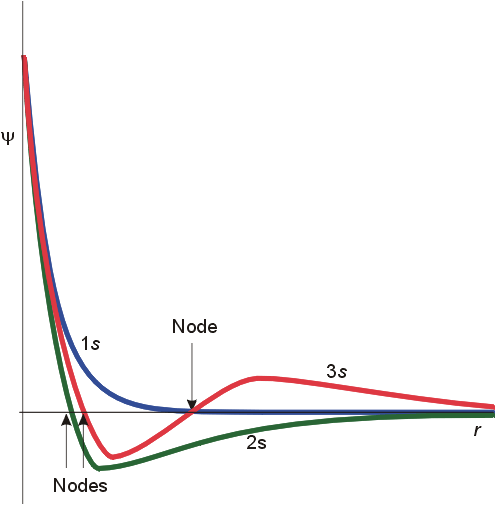
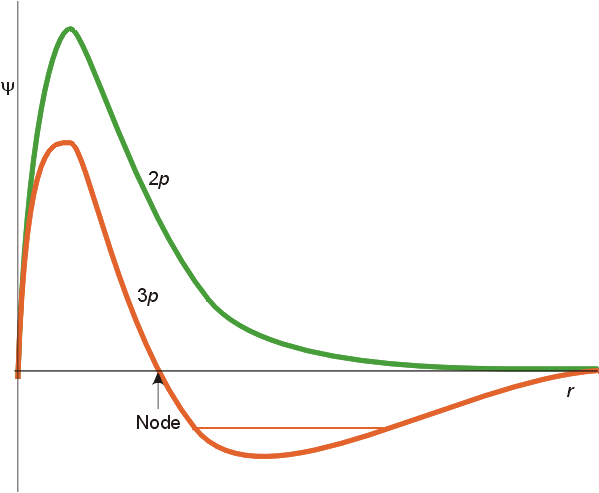
The important differences are in the finite amplitude at the nucleus for the s orbitals, and the number of nodes for each orbital. To fully understand the differing spatial orientation and penetrating ability of s and p orbitals, the radial distribution function is used. This function illustrates the probability of finding the electron at varying distances from the nucleus. The radial distribution functions for a 2s and 2p orbital are illustrated below.
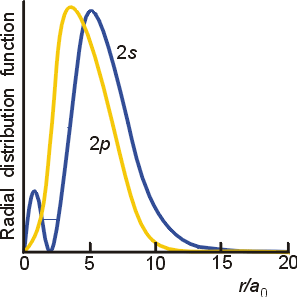
On average, the 2s electrons will be at a slightly greater distance from the nucleus than the 2p electrons. However, the 2s electrons have a higher probability of being closer to the nucleus due to the inner peak. As a result, the 2s orbital will lie lower in energy than the 2p orbital in multi-electron atoms. This is reflected in electron configurations for atoms in the second period and beyond on the periodic table.
Shielding and Effective Nuclear charge
In multi-electron
atoms, electrons are attracted to the nucleus and repelled by each other.
Consider a 3 electron atom. The third electron will experience a
lesser nuclear charge than the electrons in the 1s orbital, due to the
shielding
of the nucleus by the 1s electrons. The third electron experiences
an effective nuclear charge, Zeff, which is equal to
the total nuclear charge, Z, less the shielding, s.
Zeff =Z-s
The
extent of shielding will depend on the radial distribution (or orbital
type). Since the 2s electron has a finite probability of penetrating
the 1s orbital, there is not a large difference between the effective nuclear
charge and Z. That is, shielding is minimal. A p orbital is
less penetrating due to the nodal plane at the nucleus. (The value
of the radial distribution is zero at the nucleus.) As a result,
a p orbital is more shielded by the inner 1s orbital electrons than the
2s orbital is.
Atomic Parameters - Periodic Trends
Many atomic properties can be related to position on the periodic table. The first is electron configuration, which is directly related to an element's position in the table. [See the periodic table above.] The Aufbau Principle and Hund's Rule dictate that electrons will occupy orbitals which are lower in energy. When the orbitals are degenerate (of the same energy), the electrons will go in separate orbitals with parallel spins. The parallel spins are a result of spin correlation, which is the tendency of two electrons with parallel spins to stay apart from each other and hence repel each other less. There are some exceptions to the rules, notably in chromium and copper.
The relative sizes
of atoms or ions can be linked to position in the periodic table.
In discussion radii of atoms, the means of obtaining them must be specified.
Metallic radii are usually determined using X-ray data to determine the
inter nuclear distances in metallic crystals. Covalent radii are
half the inter nuclear distance in covalent molecules. If multiple
bonding is possible, the bond length will reflect this. Ionic radii
are determined by measuring inter nuclear distances in ionic crystals.
An arbitrary reference radius must be set for one ion, and the other ions
can be compared to the standard. Usually the oxide ion is usually
assigned a radius of 1.40 angstroms.
The general trend
is for atomic radii to decrease going across a period, and to increase
going down a group or family. In comparing the radii of elements
in the 6th period (La to Hg) to those of the 5th period, there is almost
no difference in size due to the Lanthanide contraction. As
the electrons in the f subshell are added, the value of Zeff
increases due to the added protons in the nucleus and the poor shielding
ability of the f orbitals. A similar contraction occurs in the d-block
as well.
In comparing ionic
radii, all anions are larger than their neutral atoms, and all cations
are smaller.
Copyright ©1998 Beverly J. Volicer and Steven F. Tello, UMass Lowell. You may freely edit these pages for use in a non-profit, educational setting. Please include this copyright notice on all pages.