| Graphical
Solutions for Problems |
| Chapter
2 Problems |
| Problem 2-38a. Plot of Log Co
versus Log Rate. |
 |
|
|
|
| Chapter
3 Problems |
| Problem 3-28. Plot of
bicarbonate ion concentration versus pH for the various water samples. |
 |
|
Problem 3-39. Plot of buffer capacity
versus pH for 0.01 mol L-1 silicic acid solution. Only the
first two dissociation steps are shown. |

|
| Chapter
4 Problems |
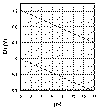
| Template for
Eh-pH diagrams. |
 |
|
Problems 40-41. Eh-pH diagram
for problems 40 and 41. Diagram drawn for [Co2+] = 10-6
mol L-1. Construction lines are shown on the diagram. |

|
| Chapter
5 Problems |
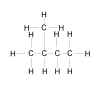
| Problem 5-39. Structural
formula for 2-methylbutane. |
 |
|
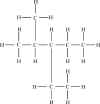
Problem 5-41. Structural formula for
3-ethyl-2-methylpentane. |
 |
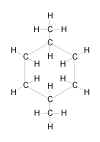
| Problem 5-42. Structural
formula for 1,4-dimethylcyclohexane. |
 |
|
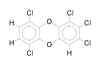
Problem 5-48. Structural formula for
1,2,3,6,9-pentachlorodibenzo-p-dioxin. |
 |
| Problem 5-52. Plot of C/N
ratios versus d13C for
various sediment samples from the Columbia river and continental margin.
Two mixing lines are drawn, one between the reservoirs marine plankton
and soil organic matter and the other between the reservoirs marine
plankton and vascular plant material. Percent of these end members in
each sample is estimated from these mixing lines. Note the marked
correlation between sediment type and end member reservoirs. The end
member reservoirs are taken from Figure 5-26 in the text. |
 |
| Chapter
6 Problems |
| Problem 6-40. Plot of age of
groundwater sample versus distance from the Danube river. |
 |
|
Problem 6-43. Plot of the natural log of the
activity versus depth. The straight-line relationship indicates a
constant sedimentation rate. |
 |
| Problem 6-44a. Plot of the
natural log of the activity versus depth for Nainital lake. The
straight-line relationship indicates a constant sedimentation rate. |
 |
|
Problem 6-44b. Plot of the natural log of
the activity versus depth for Sattal lake. The straight-line
relationship indicates a constant sedimentation rate. |
 |
| Problem 6-54. Graphical
solution for problem 6-54. G = groundwater, F = runoff from fields, R =
runoff from feedlot, and W = contaminated well water. |
 |
|
|
|
| Chapter
7 Problems |
| Problem 7-52a. Plot of Cads
versus Csoln. |
 |
|
Problem 7-52b. Plot of log Cads
versus log Csoln. The slope of the line gives the values for n
and the y-intercept gives the value for K. |
 |
| Problem 7-54a. Plot of Cads
versus Csoln. The shape of the curve, particularly the
flattening of the curve at high concentrations, suggests that the data
fit a Langmuir isotherm. |
 |
|
Problem 7-54b. Plot of 1/Cads
versus 1/Csoln. The slope of the line = 1/KQo and
the y-intercept = 1/Qo. |
 |
| Chapter
8 Problems |
| Problem 8-88. Plot of 208Pb/206Pb
versus 206Pb/207Pb for various sources and urban
aerosols in Northwestern France. |
 |
|
|
|
| Problem 8-91a. Plot of
enrichment factors, using bulk crust, for Zn and Pb versus age. |
 |
|
Problem 8-91b. Plot of enrichment
factors, using upper crust, for Zn and Pb versus age. |
 |
| Chapter
9 Problems |
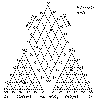
| Problem 9-77. Piper
diagram for Colorado and Nile river waters. |
 |
|
Problem 9-78. Piper diagram showing
mixing between a tributary and a river. |
 |
| Problem 9-106a. Plot of
Cl/Br ratio versus distance of groundwater sample from the petrol
station. The low Cl/Br ratios are indicative of groundwater
samples contaminated by ethylene dibromide. The higher Cl/Br
ratios are indicative of uncontaminated groundwater. |
 |
|
Problem 9-106b. Plot of Br-
versus Cl-. The data form a linear array which suggests that
the groundwater chemistry can be explained by simple mixing between
uncontaminated groundwater and contaminated groundwater from the petrol
station. |
 |