Mechanical transition from \(\alpha\)-helical coiled coils to \(\beta\)-sheets in fibrin(ogen)¶
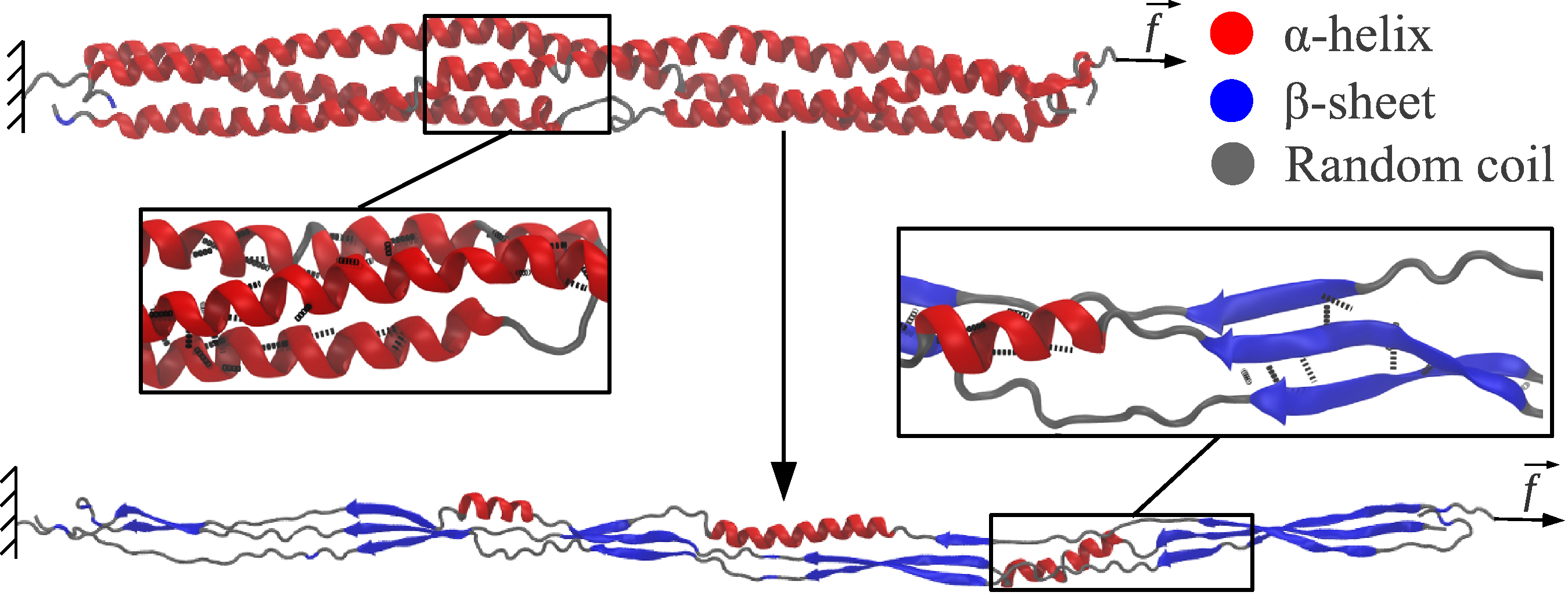
Large structural transitions constitute an essential mechanism of protein function and dysfunction, including folding, unfolding, and misfolding, conformational activation and inactivation, aggregation, protein−protein interactions, etc. Secondary structure alterations, including the \(\alpha\)-helix to \(\beta\)-sheet (\(\alpha\)-to-\(\beta\)) transition, have been suggested as a universal mechanism of protein unfolding. It has been shown experimentally and theoretically that upon mechanical unfolding, protein polymers containing two-stranded \(\alpha\)-helical coiled coils, such as \(\alpha\)-keratin, \(\alpha\)-keratin-like intermediate filaments, wool fibers, vimentin, and desmin intermediate filaments, form structures with \(\beta\)-sheet architecture. In the \(\alpha\)-helical coiled coil, two or more right-handed \(\alpha\)-helices wind around each other to form a left-handed supercoil.
We characterized the \(\alpha\)-to-\(\beta\) transition in \(\alpha\)-helical coiled-coil connectors of the human fibrin(ogen) molecule using biomolecular simulations of their forced elongation and theoretical modeling. The force (\(F\))–extension (\(X\)) profiles show three distinct regimes: (1) the elastic regime, in which the coiled coils act as entropic springs (\(F < 100-125\) pN; \(X < 7-8\) nm); (2) the constant-force plastic regime, characterized by a force-plateau (\(F \approx 150\) pN; \(X \approx 10-35\) nm); and (3) the nonlinear regime (\(F > 175-200\) pN; \(X > 40-50\) nm). In the plastic regime, the three-stranded \(\alpha\)-helices undergo a noncooperative phase transition to form parallel three-stranded \(\beta\)-sheets. The critical extension of the \(\alpha\)-helices is \(0.25\) nm, and the energy difference between the \(\alpha\)-helices and \(\beta\)-sheets is \(4.9\) kcal/mol per helical pitch. The soft \(\alpha\)-to-\(\beta\) phase transition in coiled coils might be a universal mechanism underlying mechanical properties of filamentous \(\alpha\)-helical proteins.
The results was published in J. Am. Chem. Soc. (2012).