Fuqiang Liu Group
Research
-
Core-shell Bimetallic Nanoparticles
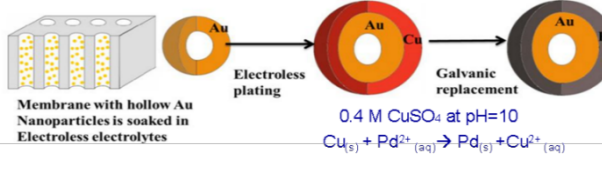
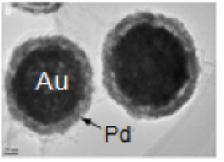
Core-shell bimetallic nanoparticles with Pd or Pt coated on hollow Au spheres (in collaboration with Dr. Yaowu Hao in MSE at University of Texas Arlington) have shown long-range electronic interaction between the cores and shells. Significant enhancement of catalytic activities of such nanoparticles toward electrochemical reactions, such as formic acid oxidation, has been demonstrated. Electrochemical stripping techniques using carbon-monoxide and formic acid showed lower onset oxidation potential of these catalysts. Most importantly, in these nanoparticles many structural parameters (shell thickness, total particle size, surface roughness, interface between the catalysts and Au layers, etc) can be independently controlled.
- Hsu, Chiajen, Mingsheng Wei, Zi Wei, and Fuqiang Liu. "Improving the Catalytic Activity of Au/Pd Core-Shell Nanoparticles with a Tailored Pd Structure for Formic Acid Oxidation Reaction." RSC Advances 6, no. 29 (2016): 24645-50.
- Hsu, Chiajen, Chienwen Huang, Yaowu Hao and Fuqiang Liu. "Electro-Oxidation of Formate-Based Solutions on Au/Pd Core Shell Nanoparticles Experiment and Simulation." International Journal of Hydrogen Energy 38 (35), (2013): 15532-15541.
- Hsu, Chiajen, Chienwen Huang, Yaowu Hao and Fuqiang Liu. "Au/Pd Core-Shell Nanoparticles with Varied Hollow Au Cores for Enhanced Formic Acid Oxidation." Nanoscale Research Letters 8, (2013): 113.
- Hsu, Chiajen, Chienwen Huang, Yaowu Hao and Fuqiang Liu. "Impact of Surface Roughness of Au Core in Au/Pd Core Shell Nanoparticles toward Formic Acid Oxidation Experiment and Simulation." Journal of Power Sources 243, (2013): 343-349.
- Chiajen Hsu, Chienwen Huang, Yaowu Hao, and Fuqiang Liu, "Synthesis of Highly Active and Stable Au/PtCu Core-shell Nanoparticles for Oxygen Reduction Reaction", Physical Chemistry Chemical Physics, 14, 14696-14701, (2012)
- Hsu, C., Huang C., Y. Hao, and Fuqiang Liu, "Au/Pd core shell nanoparticles for enhanced electrocatalytic activity and durability." Electrochemistry Communications (2012) 23 (0): 133
-
Guanidinium-based Membranes for Electrochemical Cells
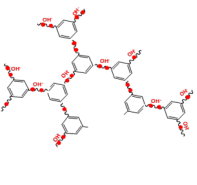
This research is to understand fundamental reaction kinetics and process issues in hydrogen production using guanidinium-based alkaline electrolytic cells. Hydrogen production from electrolysis of water has been considered as one of the most efficient and reliable approaches for renewable energy storage such as solar, wind, and hydropower; therefore, it holds a unique position in our long-term energy sustainability.Guanidinium group, with a typical structure as -C(NR)3, is a promising superbase and potentially has higher ionic conductivity than quaternary-ammonium-based membranes. We have successfully synthesized a crosslinked guanidine polymer with ion exchange groups (guanidinium groups) tethered into the polymer backbone. The positive charge is delocalized over one carbon and three nitrogen atoms, significantly alleviating the electrophilicity. High mechanical strength can be also achieved by micro-reinforcement, in which a foreign porous substrate film provides membrane integrity and the hydroxide ions are conducted through the impregnated composite membrane.
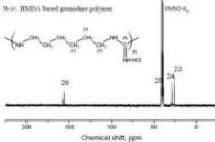
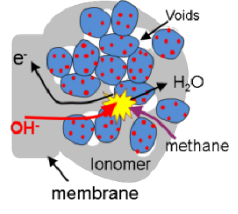
S. D. Sajjad, D. Liu, W. Zi, S. Sakri, Y. Shen, Y. Hong and F. Liu, “Guanidinium Based Blend Anion Exchange membranes for Direct Methanol Alkaline Fuel Cells (DMAFCs)”,Journal of Power Sources, Volume 300, 2015, Pages 95 103
Syed D Sajjad, Yi Hong, and Fuqiang Liu, “Synthesis of guanidinium-based anion exchange membranes and their stability assessment”, Polymer for Advanced Technology, (2014), 25, 108-116
Photoelectrochemical Solar Energy Storage and Conversion
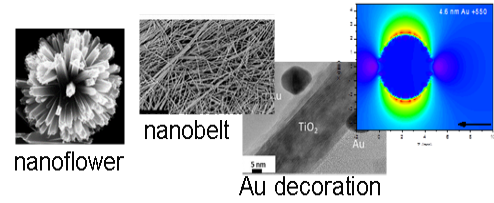
Sunlight is one of the most abundant renewable energy sources. The ability to store solar energy on a large scale and use as a renewable alternative provides a sustainable solution to the problem of energy shortage. This research is to develop, for the first time, a new type of solar energy conversion process that could potentially store massive amount of solar energy using photoelectrochemical redox pairs. This approach is based on an all-vanadium photoelectrochemical cell in which advanced semiconductors are in contact with vanadium redox species. Under sunlight, the electrons and holes created by the semiconductors react photoelectrochemically with different vanadium species similar to the way a rechargeable battery works. The process stores the solar energy. This design takes advantage of highly reversible redox-couple reactants such as vanadium ions that remain in the liquid during both photocharge and discharge, which are advantageous for higher capacity and efficiency. Besides, when combined with a hybrid electrode (shown in the figure below), this system could offer instant high-capacity electron storage and significant improvement in the photocurrent.
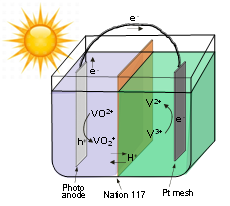
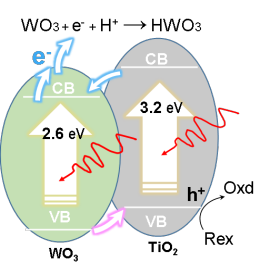
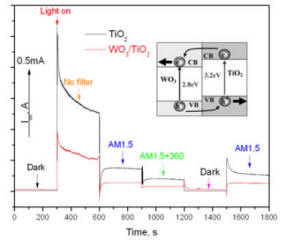

Wei, Zi, Yi Shen, Dong Liu, Chiajen Hsu, Syed D. Sajjad, Krishnan Rajeshwar, and Fuqiang Liu. "Geometry-Enhanced Ultra-Long TiO2 Nanobelts in an All-Vanadium Photoelectrochemical Cell for Efficient Storage of Solar Energy." Nano Energy 26 (8// 2016): 200-07.
D. Liu, Z. Wei, Y. Shen, S. D. Sajjad, Y. Hao and F. Liu, “Ultra-long Electron Lifetime Induced Efficient Solar Energy Storage by an All-Vanadium Photoelectrochemical Storage Cell Using Methanesulfonic Acid”, J. Mater. Chem. A, 2015,3, 20322-20329
Dong Liu, Wei Zi, Syed D. Sajjad, Chiajen Hsu, Yi Shen, Mingsheng Wei, and Fuqiang Liu, “Reversible Electron Storage in an All-Vanadium Photoelectrochemical Storage Cell: Synergy between Vanadium Redox and Hybrid Photocatalyst”, ACS Catalysis, 5, (2015) 2632-2639
Dong Liu, Zi Wei, Chiajen Hsu, Yi Shen, and Fuqiang Liu, “Efficient Solar Energy Storage Using A TiO2/WO3 Tandem Photoelectrode in An All-vanadium Photoelectrochemical Cell”, Electrochimica Acta, 136 (2014): 435-441
Chiajen Hsu, Shen Yi, Wei Zi, Dong Liu and Fuqiang Liu, “Anatase TiO2 nanobelts with plasmonic Au decoration exhibiting efficient charge separation and enhanced activity”, Journal of Compounds and Alloys, 613 (2014): 117-121
Zi Wei, Dong Liu, Chiajen Hsu, and Fuqiang LIu, “All-vanadium redox photoelectrochemical cell: An approach to store solar energy”, Electrochemistry Communications, 45 (2014) 79-82