Approaches
We use a variety of techniques to study the biochemical and mechanical properties of molecular motors, the cytoskeleton and biopolymers.
• Total internal reflectance fluorescence microscopy
• Cytoskeletal Network Rheology
• Unloaded in vitro motility assay
• Frictional loading in vitro motility assay
• Optical-Tweezer Active Microrheology
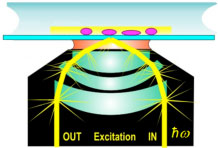
Total internal reflectance fluorescence microscopy
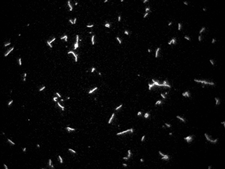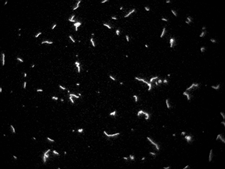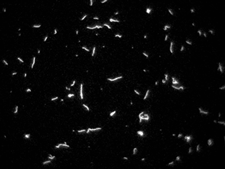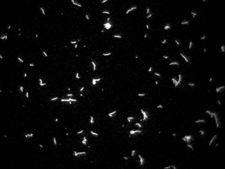
TIRF microscopy is achieved by aiming an excitation laser onto a microscope glass slide beyond the critical angle of refraction. This can be done either through a microscope objective, as shown on the picture, or through a glass prism. In either case, the resulting evanescent wave excites fluorophores only very near the glass surface and greatly reduces fluorescent background from fluorophore in solution. This makes assays with large amounts of labeled protein in solution possible, such as assembly assays of labeled G-actin into F-actin filaments.
Confocal Microscopy
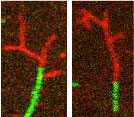 We use confocal microscopy to study how various actin binding proteins regulate filament dynamics and interactions with other actin binding proteins. For example, we recently demonstrated that caldesmon-stabilized nascent actin filaments more readily bind Arp2/3 and form branched structures.
We use confocal microscopy to study how various actin binding proteins regulate filament dynamics and interactions with other actin binding proteins. For example, we recently demonstrated that caldesmon-stabilized nascent actin filaments more readily bind Arp2/3 and form branched structures.
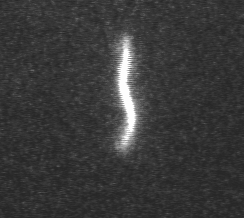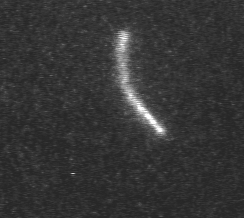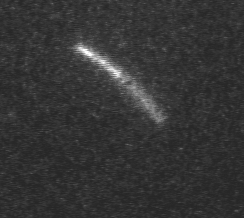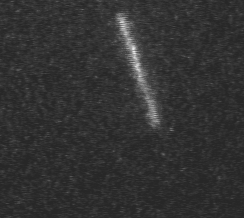 Actin filament mechanics
Actin filament mechanics
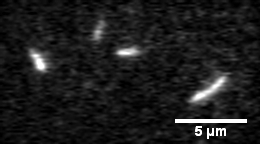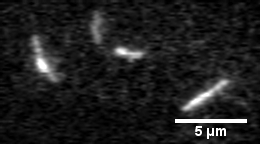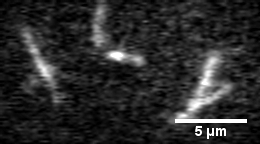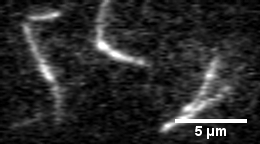
In this assay individual fluorescently labeled actin filaments are observed via fluorescence microscopy. Filament motion is constrained to two dimensions by constructing a narrow observation chamber and recorded with an ICCD camera. We use the thermal induced shape changes to quantify actin filament persistence length.
Cytoskeletal Network Rheology
 In this assay we generate defined cross-linked networks of actin and actin binding proteins. We measure their mechanical properties using bulk rheology in collaboration with the Weitz lab at Harvard University. Recent work aims to elucidate the effects of calponin and tropomyosin in this system, which more closely mimicking a cytoskeletal actin network but can be more precisely controlled experimentally.
In this assay we generate defined cross-linked networks of actin and actin binding proteins. We measure their mechanical properties using bulk rheology in collaboration with the Weitz lab at Harvard University. Recent work aims to elucidate the effects of calponin and tropomyosin in this system, which more closely mimicking a cytoskeletal actin network but can be more precisely controlled experimentally.
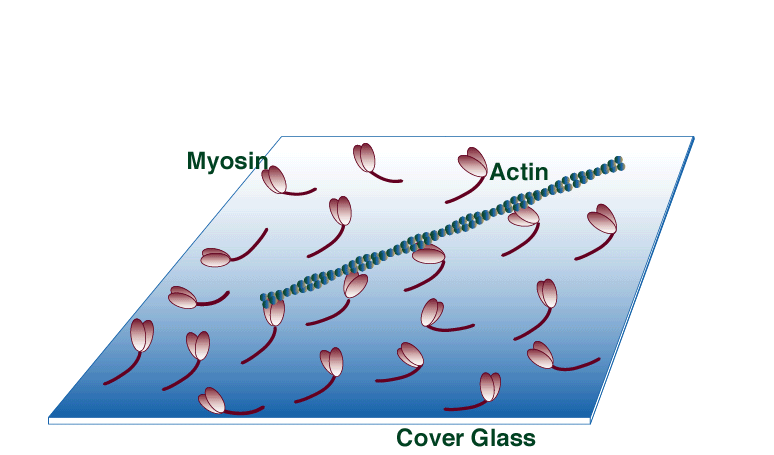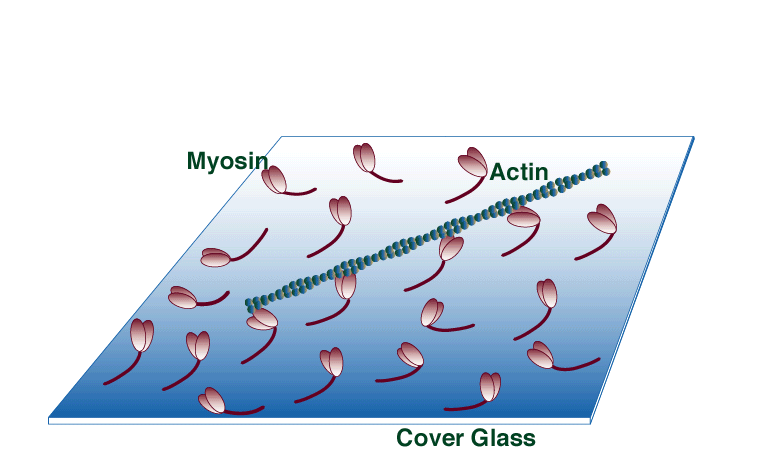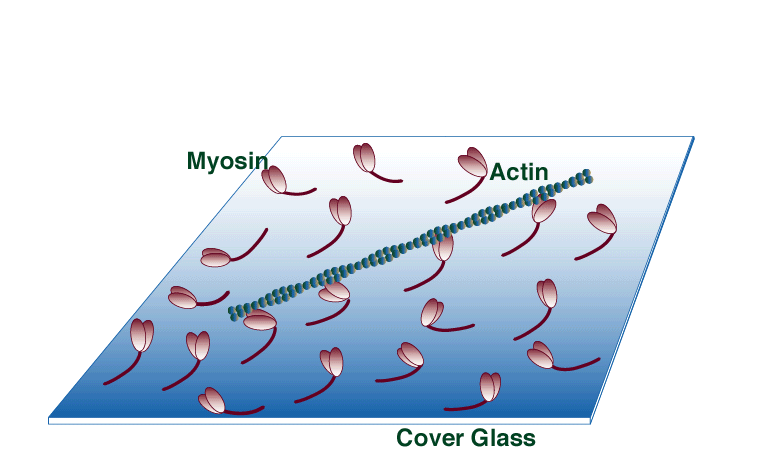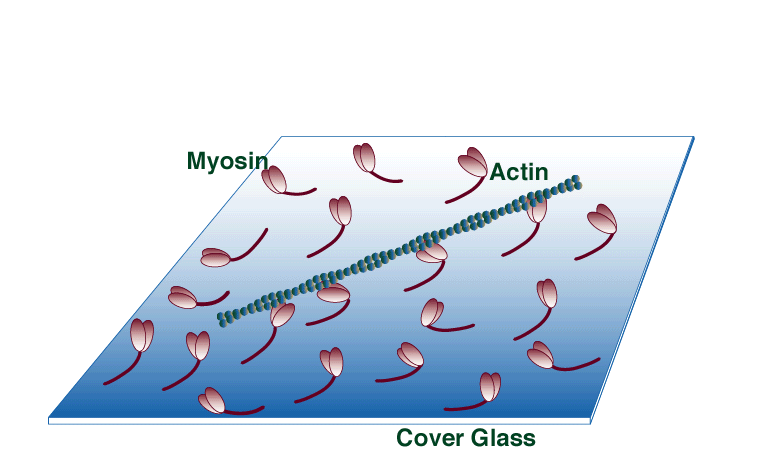
Unloaded in vitro motility assay
The unloaded in vitro motility assay has all the advantages of solution biochemistry (i.e., temp, ionic conditions, substrate concentrations etc. are easily manipulated); however, movement, one of the fundamental properties of molecular motor function, can also be quantified. In this assay myosin is adsorbed to a silanized coverslip surface. Fluorescently labeled actin filaments are then allowed to bind to the surface-bound myosin. Movement is initiated by the addition of ATP and recorded with an ICCD camera.
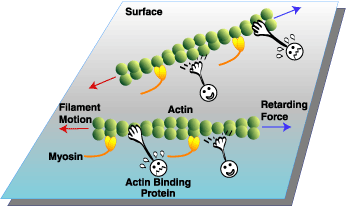 Frictional loading in vitro motility assay
Frictional loading in vitro motility assay
The unloaded motility assay gives important information about the motion generating capacity of molecular motors. However, motor molecules also produce force. We assess the effects of myosin or thin filament regulatory protein mutations on relative (to wild-type) force generation. In this assay, load is applied to the myosin motor by applying it to the surface along with small amounts of non motile actin binding proteins (e.g., alpha-actinin). The alpha-actinin will introduce a frictional load that resists filament motion. The amount of alpha-actinin required to stop filament motion is an indicator of myosin force generating capability. Since the load is applied to all of the filaments in a field of view, this assay is useful for rapidly screening a variety of experimental conditions (e. g.: regulated actin, unregulated actin, submaximal calcium activation, etc.).
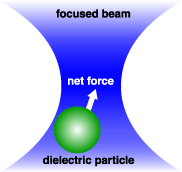 Optical trap
Optical trap
Optical traps use photon momentum to capture and hold small transparent particles with a higher refractive index than the medium. As a trapped particle moves away form the laser focus there is a restoring force that is proportional to the distance the particle moves. Therefore, an optical trap can be used as a very sensitive force tranducer that can measure forces in the piconewton range.
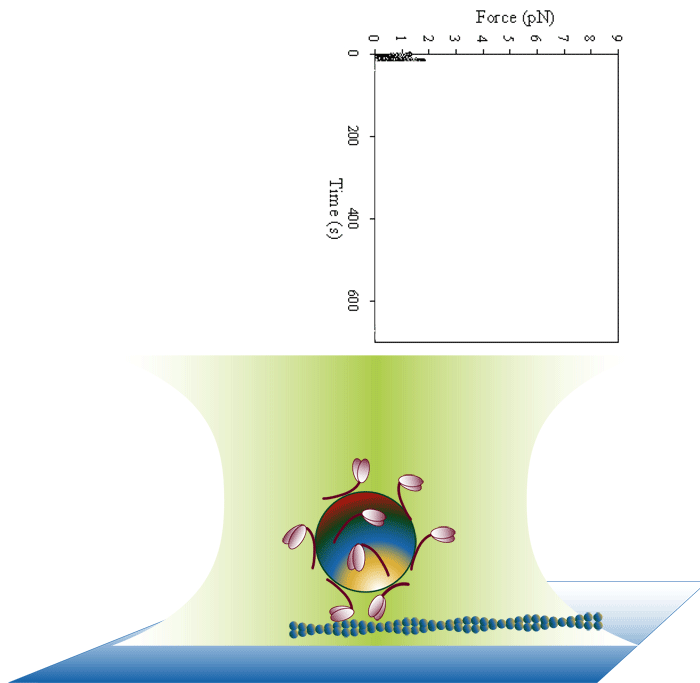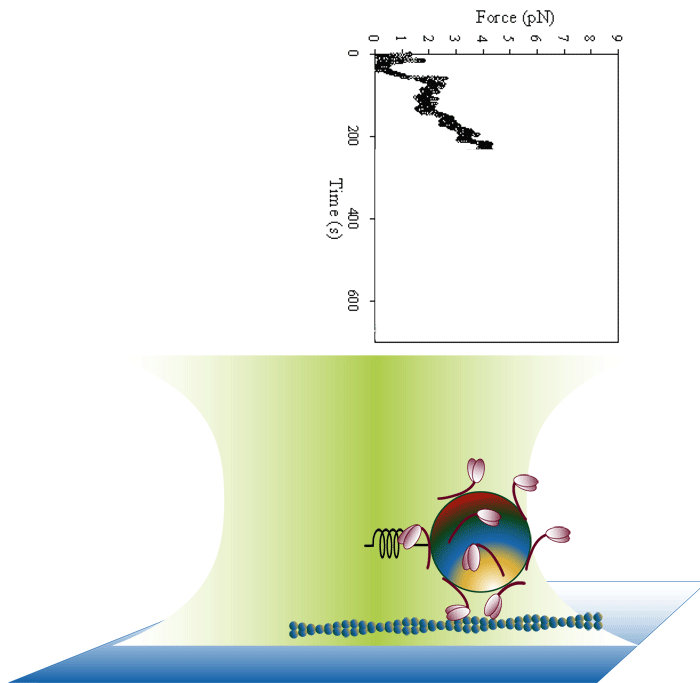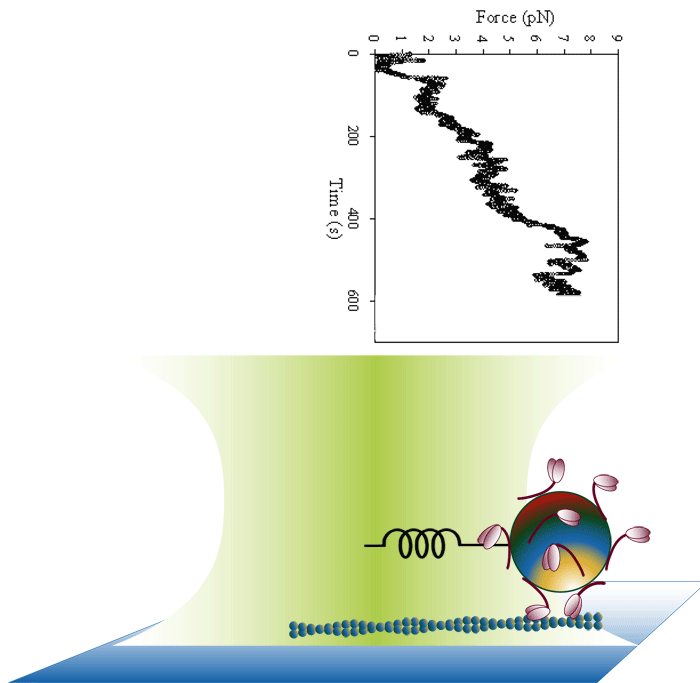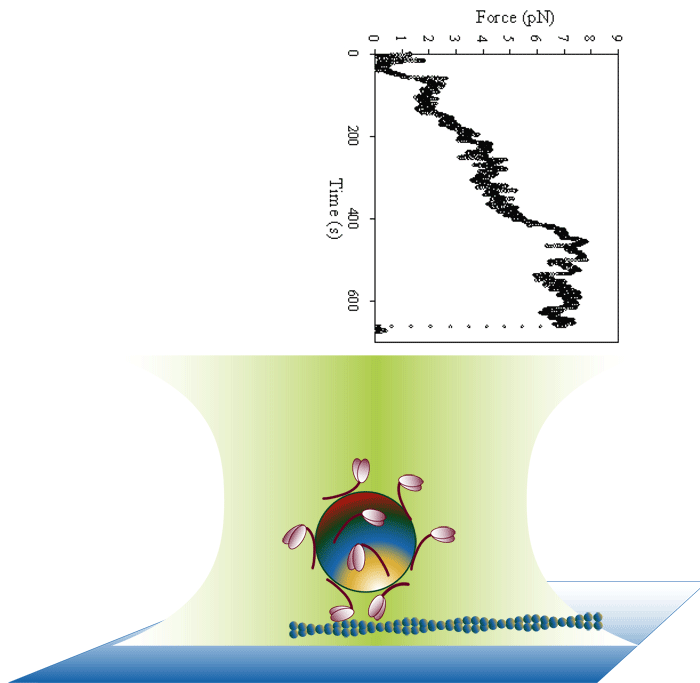 Laser trap loading assay
Laser trap loading assay
We use both optical trap and frictional loading based assays to measure the inherent force generating capacity of mutant myosin molecules. Frictional loading assays provide an indication of relative force while optical trap based assays measure the absolute isometric force generating capability of the myosin mutants one filament at a time.
Optical-Tweezer Active Microrheology
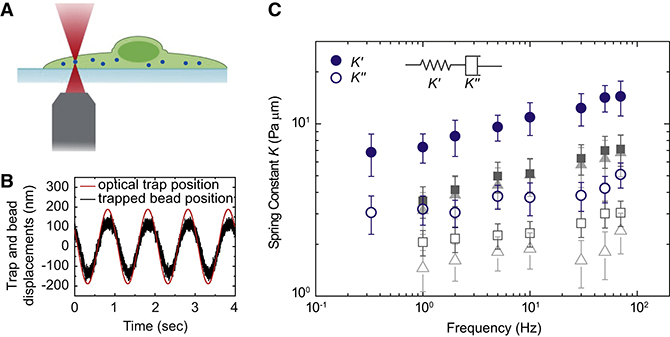 In addition to bulk rheology we also measure the microrheology of cross-linked networks of actin and actin binding proteins. In this method we use optical tweezers to apply forces and derive the stress/strain relationship. Measurements of intracellular mechanical properties shows that the cytoplasm behaves as a weak elastic gel. For these measurements the optical trap position is oscillated sinusoidally over a range of frequencies. The relationship between the amplitude and phase of the driving laser position and the position of an intracellular particle are used to determine the in-phase (elastic) and out of phase (viscous) moduli. Our measurements show that the intracellular elastic stiffness dominates over the dissipative resistance.
In addition to bulk rheology we also measure the microrheology of cross-linked networks of actin and actin binding proteins. In this method we use optical tweezers to apply forces and derive the stress/strain relationship. Measurements of intracellular mechanical properties shows that the cytoplasm behaves as a weak elastic gel. For these measurements the optical trap position is oscillated sinusoidally over a range of frequencies. The relationship between the amplitude and phase of the driving laser position and the position of an intracellular particle are used to determine the in-phase (elastic) and out of phase (viscous) moduli. Our measurements show that the intracellular elastic stiffness dominates over the dissipative resistance.