Research
Research Overview
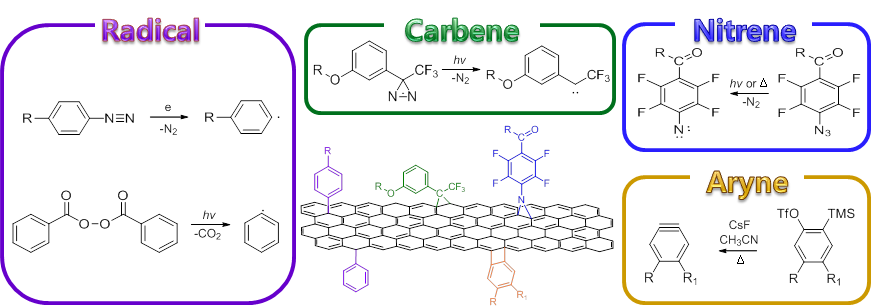
Research in our laboratory has a general focus on developing new conjugation chemistry that is general, efficient, can accommodate ligand diversity, maintain ligand bioaffinity, and give bioactive and stable interfaces. We develop efficient conjugation reactions, and apply them to synthesize functional surfaces and nanomaterials to solve biointerface problems. We have studied extensively the reactivities of perfluoroaryl azides, as highly reactive singlet nitrene precursors, as well as electron-deficient azides in metal catalyst-free cycloaddition reactions. We use these reactions to functionalize graphene, to screen for antifouling polymers, to fabricate glycan microarrays and screen carbohydrate-binding lectins and bacteria, to synthesize glyconanomaterials to image and target bacteria.
Students in our lab have the opportunity to be trained in organic synthesis, nanomaterials synthesis, functionalization and characterization, and in vistro bioassays. Students graduated from our laboratory have found employment in academia (universities, national labs) and industrial sectors (semiconductor, biotechnology, chemical analysis).
Covalent functionalization of pristine graphene
The unique and superior properties of graphene make it the nanomaterial of choice for a broad range of applications. Despite its high potentials, major challenges remain, including poor solubility, intrinsic zero band gap energy, low reactivity and the availability of well-defined pristine graphene in large quantity, which have hampered the rapid development of graphene-based functional devices. Many of these challenges can be potentially addressed through chemical functionalization of the material. Pristine graphene has its pi electrons delocalized over the entire 2D network, and is thus fairly inert chemically. We use a reactive intermediate, singlet perfluorophenyl nitrene, generated from activating perfluorophenyl azide (PFPA) by heat, microwave, or UV, to functionalize pristine graphene. By changing the functional group on PFPA, we could make the graphene soluble in common organic solvents as well as in water.
Liu, L.-H.; Yan, M. A Simple Method for the Covalent Immobilization of Graphene, (2009) Nano Lett. 9, 3375.
Liu, L.-H.; Lerner, M. M.; Yan, M. Derivatization of Pristine Graphene with Well-defined Chemical Functionalities, (2010) Nano Lett. 10, 3754. (Highlighted in (2010) Nature, 466, 904.)
Liu, L.-H., Yan, M. Perfluorophenyl Azides: New Applications in Materials Synthesis and Surface Functionalization, (2010) Acc. Chem. Res. 43, 1434.
Park, J.; Yan, M. Functionalization of Graphene with Reactive Intermediates, (2013) Acc. Chem. Res. 46, 181.
Park, J.; Jaywawadena, H. S. N.; Chen, X.; Jayawadana, K.; Sundhoro, M.; Ada, E.; Yan, M. A General Method for the Fabrication of Graphene-Nanoparticle Hybrid Material, (2015) Chem. Commun. 51, 2882.
Perfluoroaryl azides (PFAAs) as electrophilically activated dipoles
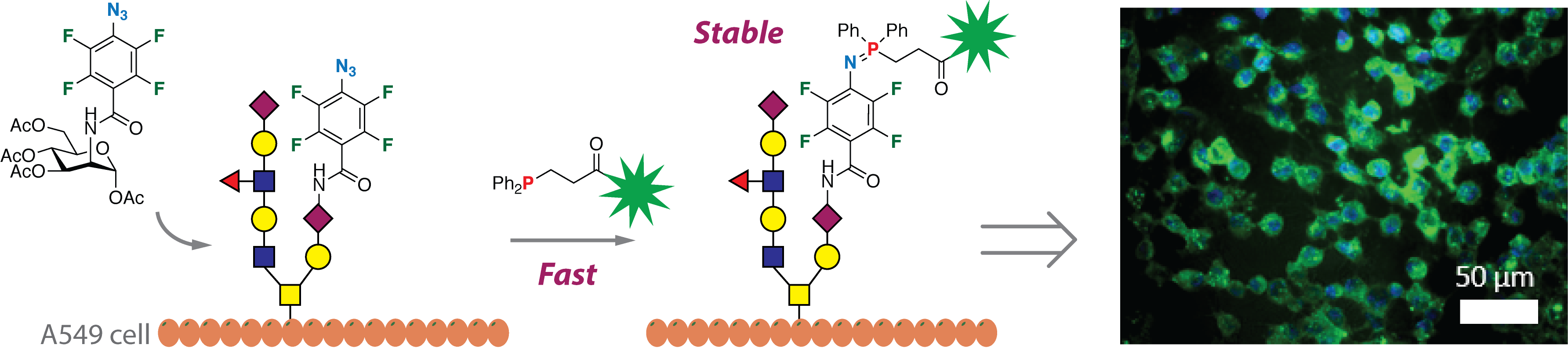
We developed a series of new reactions taking advantage of the high electron-deficient nature of PFAAs. The F atoms on the phenyl azide lower the LUMO of PFAA, thus the reactions with nucleophiles or dipolarophiles occur at room temperature in high yields without the use of any metal catalysts. These reactions are now been applied to synthesize glyconanomaterials and drug conjugates.
Xie, S.; Lopez, S.; Ramstrom, O.; Yan, M.; Houk, K. N. 1,3-Dipolar Cycloaddition Reactivities of Perfluorinated Aryl Azides with Enamines and Strained Dipolarophiles, (2015) J. Am. Chem. Soc., 137, 2958.
Xie, S.; Zhang, Y.; Ramstrom, O.; Yan, M. Base-catalyzed synthesis of aryl amides from aryl azides and aldehydes, (2016) Chem. Sci., 7, 713.
Sundhoro, M.; Jeon, S.; Hao, N.; Park, J.; Ramstrom, O.; Yan, M. Perfluoroaryl Azide–Staudinger Reaction: A Fast and Bioorthogonal Reaction, (2017) Angew. Chem. Int. Ed, 56, 12117.
Glyconanomaterials
Carbohydrates are involved in a wide range of biological processes including cell communication and bacteria infections. Carbohydrate-decorated nanomaterials, i.e., glyconanomterials, could be envisioned as cell-mimics, having potential to be used for imaging, diagnostics and therapeutics. Our goal is to develop conjugation chemistries and analytical methods to synthesize and characterize glyconanomterials, and applying them in treating bacterial infections. We have thus developed versatile coupling chemistry for the synthesis of glyconanomaterials that applies to mono-, oligo-, and poly-saccharides, reducing and non-reducing sugars, derivatized and un-derivatized carbohydrates. We developed 4 different methods to determine the binding affinity of glyconanoparticle-lectin interactions, including i) fluorescence competition assay, ii) dynamic light scattering, ii) isothermal titration calorimetry, iv) super-microarray. We conducted comprehensive studies on the impact of ligand presentation on glycan-lectin interactions. Our results demonstrate that the binding affinity of glyconanomaterials is highly influenced by how the ligands are presented on the surface. The results pave the way to tailor-make glyconanomaterials with tunable affinity by ligand display.
Wang, X.; Ramstrom, O.; Yan, M. Glyconanomaterials: Synthesis, Characterization, and Ligand Presentation, (2010) Adv. Mater. 22, 1946.
Wang, X.; Ramstrom, O.; Yan, M. Quantitative Analysis of Multivalent Ligand Presentation on Gold Glyconanoparticles and the Impact on Lectin Binding, (2010) Anal. Chem. 82, 9082.
Chen, X.; Ramstrom, O.; Yan, M. Glyconanomaterials: Emerging applications in biomedical research, (2014) Nano Res. 7, 1381.
Hao, N.; Neranon, K.; Ramstrom, O.; Yan, M. Glyconanomaterials for Biosensing Applications, Biosens. Bioelectron. 2016, 76, 113.
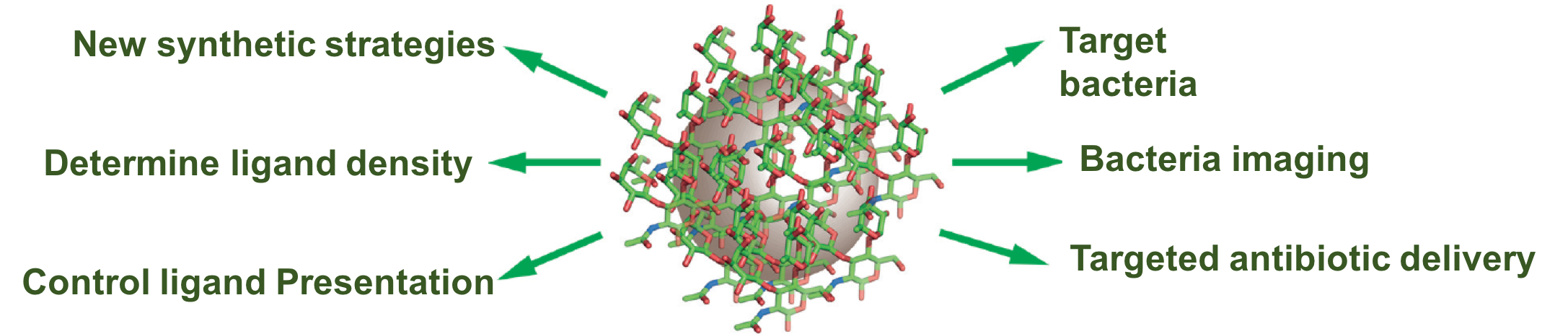
Nanoantibiotics
Antimicrobial resistance (AMR) has spread rapidly around the globe, making it an increasing threat to the society. Once powerful antibiotics have now become virtually useless. The situation is especially dire for Gram-negative bacteria where the vast majority of antibiotics are no longer effective. We are developing new strategies that help antibiotics to traverse penetration barriers and accumulate, and thus revive and extend the lifespan of antibiotics. For example, we discovered that certain sugars, when conjugated onto nanoparticles, could significantly increase the surface binding and penetration of nanoparticles into the bacterium. These are bacterium-specific sugars as they are important for the survival of the bacterium but are not expressed by human cells. We are also developing antibiotic-conjugates and nanodrugs to combat AMR.
Hao, N.; Chen, X.; Jeon, S.; Yan, M. Hollow Oblate Mesoporous Silica Nanoparticles: Synthesis and Target Antibiotic Delivery to Mycobacteria, Adv. Healthc. Mater. 2015, 4, 2797.
Chen, X.; Wu, B.; Jayawadena, K.; Hao, N.; Jayawadena, H. S. N.; Langer, R.; Jaklenec, A.; Yan, M. Magnetic multivalent trehalose glycopolymer nanoparticles for the detection of mycobacteria, Adv. Healthc. Mater. 2016, 5, 2007.
Xie, S.; Manuguri, S.; Proietti, G.; Romson, J.; Fu, Y.; Inge, A. K.; Wu, B.; Zhang, Y.; Hall, D.; Ramstrom, O.; Yan, M. Design and Synthesis of Theranostic Antibiotic Nanodrugs that Display Enhanced Antibacterial Activity and Luminescence, (2017) Proc. Natl. Acad. Sci. USA , 114, 8464.
