Transition from “catch” bonds to “slip” bonds in cell-adhesion systems¶
Biological adhesion is at the intersection of material science, biology, and nanotechnology. It turns out that the bond lifetimes of some of the most biologically important adhesive bonds, called “catch” bonds, are enhanced mechanically, and only when shear stress exceeds some critical value (i.e. shear threshold) these bonds become weeker and break (“slip” bonds).
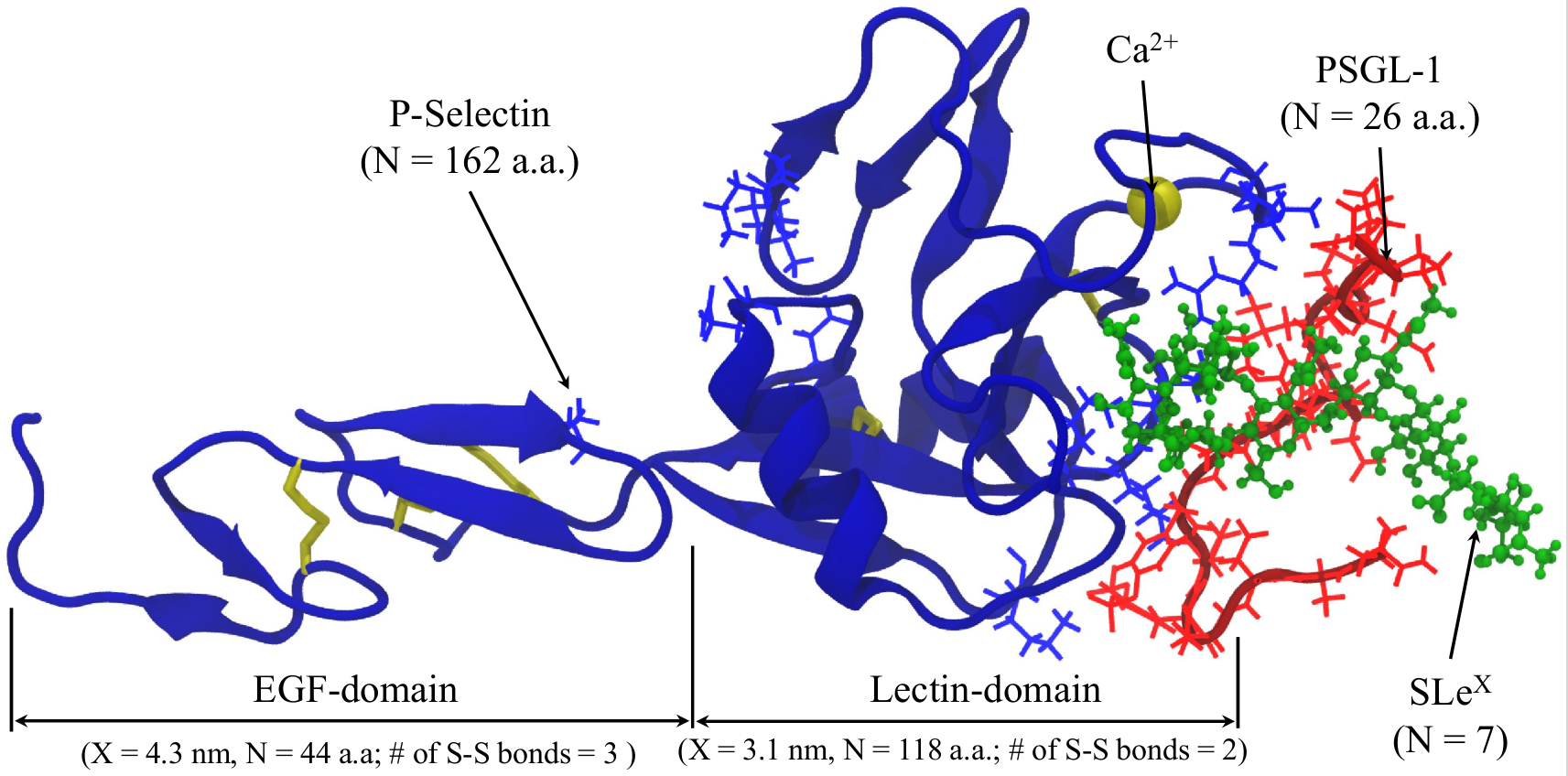
P-selectin – PSGL-1 complex¶
P-selectins, a member of the selectin family of adhesion proteins, are known to facilitate the leukocyte rolling upon the in ammatory response. It was shown experimentally, that under the external force application the bond between P-selectins and their complementary ligand, P-selectin glycoprotein-1 (PSGL-1), exhibit non-standard behavior. When the external force increases, the P-selectin – PSGL-1 bond lifetime initially increases, and only then decreases.
We utilized computational acceleration on Graphics Processing Units (GPU) in conjunction with implicit solvent models to resolve the structural details of the second bound state in the P-selectin – PSGL-1 bond dissociation profile. The combination of the implicit solvent approach with the GPU acceleration allowed us to follow the dynamics of the system in \(1-2 \mu s\) timescale. We observe, that in some cases, the number of contacts between P-selectin and PSGL-1 initially increased with the increasing force, which was shown to be due to the structural transition at the microscopic level. Specifically, the loop containing amino acids Asn57-Glu74 in lectin domain dissociates from the EGF-domain and associates with the PSGL-1 ligand. It was suggested that formation of the “catch” bond is mediated by the exibility of the hinge region between the EGF domain and the lectin domain of the P-selectin receptor. The Asn57-Glu74 loop is bound to this hinge region of the P-selectin receptor in its native state. The observed bending motion can be shown to be directly related to the fluctuating strength of interactions that hold the Asn57-Glu74 loop bound to the EGF domain in the native state. Hence, when the receptor is stretched, these interactions become stronger constraining the position of the loop. As a result, the strength of the bond does not change (“slip” bond). When the external force is low, the loop is relatively free to move and can switch its binding contacts from the EGF domain to the PSGL-1 ligand, thus, mediating formation of the “catch” bond. This dynamic behavior dominates the dissociation kinetics until the mechanical force reaches some characteristic force at which all the cryptic contacts buried in the receptor-ligand interface in the unstretched state are fully engaged. Above the characteristic force, these newly emerging interactions become weaker and the dissociation kinetics enters the “slip” regime of bond rupture.